usp class vi materials
USP Class VI materials meet the most stringent requirements and include silicones that pass a systemic toxicity test an intracutaneous test and an implantation test. Its possible that a USP Class VI material can also comply ISO 10993.
In 1988 in vitro tests were explored and USP concluded that in vitro.

. Pharmacopoeia USP Class VI outlines requirements for system toxicity and intracutaneous toxicity for these cleaner compounds. Certificates of Analysis COAs report the test results for a specific batch of materials. Certified USP Class VI Silicones.
I - VI with USP Class VI being the strictest requiring that the material exhibit very low levels of toxicity proven. Yet some suppliers that use compliant ingredients may still not be able to guarantee a compliant end-product. Building trust in mRNA-based therapies.
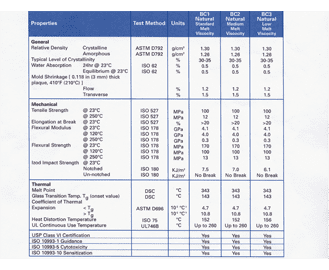
The United States Pharmacopoeia USP 30 NF 25 2007 standard also known as Class VI is widely used to comply with stringent FDA regulations for products that come in contact with the human body. This product is being manufactured and sterilized under government regulated guidelines and should cause no harmful effects to any patient. Most importantly use of Class VI certified materials substantially reduces the risk of causing harm or increased stress to a patient from reaction to a toxic material.
The intended primary audiences of this guidance. This form of testing is designed to certify that no harmful reactions or long-term issues are caused to the body by chemicals that are released or leached from plastic materials. What are Class VI materials.
The materials listed below are ideal for. The USP Class VI compounds must be made from ingredients with clear histories of biocompatibility that meet tighter requirements for leachates. Typical applications for our FDA NSF 51 USDA materials are disposable medical.
Intracutaneous tests are used to assess the localised reaction of tissue to. Its a great way to ensure youve chosen exactly what you need. 7 USP Class VI materials EPDM silicone fluorocarbon and perfluoroelastomer 24 materials which are compliant to FDA 21 CFR1772600 Specially formulated for.
Newman USP Class VI O-Rings combine. The FDA requires testing of finished devices however the demonstration of biocompatibility of materials according to USP Class VI standards is provided as an aid to device manufacturers in their. Newman designs reflect decades of experience in the design of cleanable drainable user friendly O-rings that perform day-in and day-out.
Fixes needed to prepare medicines supply chain for next crisis. Download free CADs and request free samples which are available for most of our solutions. Rigorous in-house performance testing with.
A Safety and Effectiveness. These tests correspond to numbered classes and use different extracts such sodium chloride and alcohol saline. Device is made of all USP Class VI material.
Moldable polyurethanes Resilon 4300 and 4301 Molythane 4615 Machinable polymer-filled 0618 PTFE Life Sciences Capabilites. Registered to ISO 9001 FEA design engineering Application engineering Material characterization Precision CNC machining Injection molding. Refer to biocompatibility statement enclosed.
FINAL - The purpose of this guidance document is to describe the technologies tools and methods available to owners or operators of Class VI wells to fulfill the Class VI Rule requirements related to developing and implementing site- and project-specific strategies for testing and monitoring. USP Class Testing standards are determined by the United States Pharmacopeia and National Formulary USP-NF the organization responsible for the quality and safety of medical devices and foods. Suitability under USP Class VI is typically a base requirement for medical device manufacturers.
But USP Class VI by itself is not adherence to ISO 10993. Class VI Gasket Material Options. In addition SIMONA PP-H USP Class VI sheet delivers high chemical and corrosion resistance excellent surface appearance with high durability and strength.
USP Class VI Testing is only one standard of biocompatibility however. Certificates of Conformance COC attest to a batchs. Our USP Class VI certified material offering includes.
Specialty Silicone Products SSP provides complete certifications to demonstrate the quality of its SSP-2390 Series USP Class VI FDA and RoHS compliant silicones. Among USP classes Class VI materials meet the toughest testing requirements. USP Plastic Class VI as this group is also known covers materials that pass a systemic toxicity test an intracutaneous test and an implantation test.
In order to pass the Class VI standards the productmaterial must exhibit a very low level of toxicity by passing all the tests requirements when tested according to ISO 10993. The USP outlines classes for plastic materials ie. The United States Pharmacopeia and National Formulary USP-NF determine the USP Class.
USP Class VI USP Class VI Permanent USP Class VI Prolonged USP Class IV Limited Circulating Blood USP Class VI Permanent. 1965 USP XVII introduced Biological TestsPlastics Containers section was added and made official in the Compendium. Class plastics testing is not a substitute for testing performed according to ISO standards but is often used by manufacturers to classifycertify materials.
Present in extracts of test materials. Download free CADs and try before you buy. Standards respond to public health crises.
The USP defines six plastics classes from class I to class VI with class VI being the most. Biainoompatibility andor toxicity in using this device is virtually zero. SIMONA PP-H USP Class VI sheet material is easy to clean and disinfect using most hospital grade cleaners and disinfectants.
Pharmacopoeia Class VI judges the suitability of plastic material intended for use as containers or accessories for parenteral preparations. A selection of Figure 4 VisiJet Accura and DuraForm plastic materials have met the requirements of ISO 10993-5 -10 or USP Class VI testing. Class VI testing is aimed to certify that there are no harmful reactions or long-term bodily effects caused by chemicals that leach out of plastic materials.
It generally ensures a high quality level and better acceptance with the FDA and USDA. Plastics were assigned Class I-VI based on the biological in vivo testing systemic injection intra-cutaneous and implantation tests. When forming and fabricating the machinability is highly consistent and.
Overview of USP Class VI Approved Plastic Materials USP Class VI Approved Plastic Materials USP US. Our time-tested engineered proprietary materials that are certified the world over to meet the highest industry standards. The United States Pharmacopeia USP is an independent organisation that established a set of.
As one of the most widely used methods VI forms part of six different classes with this being the most thorough. -55F -48C to 275F 135C short term to 325F 163C Processing Areas CIP Purified Water Systems Ozone-treated Water. A number of our plastic materials are ISO-10993 or USP Class VI capable.
What is the difference between USP Class VI and ISO 10993. Resists water ozone heat cold steam abrasion moderate acids and bases. 3D printing of dental and orthopedic surgical guides.
3D printing of one day crown prep guides.

Usp Class Vi And Biocompatibility Of Products For Pharmaceutical Use Mtg

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Rulon 641 Usp Approved And Fda Compliant Tristar Plastics

Material Selection Medical Injection Molding Xcentric Mold

What Is Usp Class Vi Testing Tbl Plastics

Usp Class Plastics Pacific Biolabs

Usp Class Vi Foster Corporation
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Meaning Of Usp Class Vi Standard United Kingdom
Meaning Of Usp Class Vi Standard United Kingdom
Biopharmaceutical Usp Class Vi Gaskets Newman Sanitary Gasket
Dursan Passes Usp Class Vi Testing Why Is That Important

O Rings Fda And Usp Class Vi Darcoid Rubber Company Oakland California



